Genetic Diagnostic Testing Service
Announcement: OGI Genetic Eye Disease (GEDi) Diagnostic Test Panels and Pricing Have Been Updated As of 11/1/2016.
For inquiries about Diagnostic Testing, please contact: OGI_Diagnostics@meei.harvard.edu. If you need to make an appointment or referral, call 617-573-3202.
Please note: Turn-around time is approximately 90 Days.
GENETIC EYE DISEASE (GEDi) DIAGNOSTIC TEST INFORMATION:
TESTING PANELS:
- Genetic Eye Disease Panel for Retinal Genes (GEDi-R)
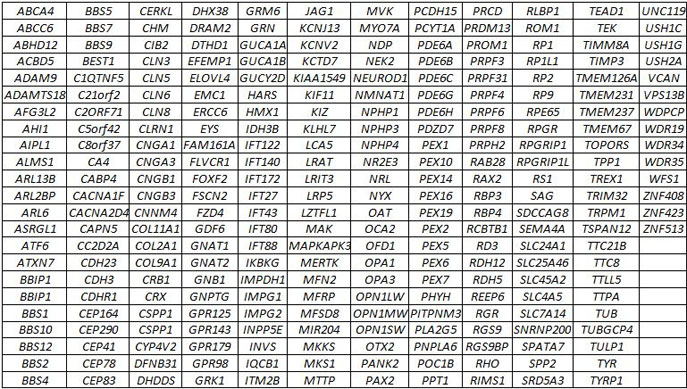
Two-hundred, sixty-seven genes (see gene list below) currently known to be involved in inherited retinal degenerations and related disorders are analyzed by a combination of SureSelect targeted enrichment, followed by Next Generation sequencing using an Illumina MiSeq instrument. This test also includes sequencing of the mitochondrial genome. The following intronic variants are analyzed: CEP290 c.2991+1655A>G; PRPF31 c.1374+654C>G; USH2A c.7595-2144A>G; OFD1 c.935+706A>G, 5 ABCA4 (c.5196+1137G>A, c.5196+1216C>A, c.5196+1056A>G, c.4539+2001G>A, c.4539+2028C>T), CHM c.315-4587T>A, SDCCAG8 c.740+356C>T, PRDM13 chr6:100040906G>T; chr6:100040987G>C; chr6:100041040C>T.
- Genetic Eye Disease Panel for Optic Nerve Disease and Early Onset Glaucoma (GEDi-O)
Twenty-two genes (ACO2, AFG3L2, AUH, C12orf65, CISD2, CYP1B1, FOXC1, FOXF2, LTBP2, MTPAP, MYOC, NDUFS1, NR2F1, OPA1, OPA3, OPTN, PAX6, PITX2, POLG, SPG7, TEK, and WFS1) currently known to be involved in optic nerve disease, early onset glaucoma and related disorders are analyzed by a combination of SureSelect targeted enrichment, followed by Next Generation sequencing using an Illumina MiSeq instrument. Panel also includes sequencing of the mitochondrial genome.
- Genetic Eye Disease Panel for Strabismus (Gedi-S)
Eight genes (ROBO3, PHOX2A, HOXA1, SALL4, CHN1, TUBB3, KIF21A, HOXB1) currently known to involved in congenital cranial dysinnervation disorders and disorders of axon guidance analyzed by a combination of SureSelect targeted enrichment followed by Next Generation sequencing using an Illumina MiSeq instrument.
- Confirmation of Research Findings or Familial Variant Testing
Sanger sequencing is performed to confirm a genetic variant identified by research testing or confirmation of a familial variant. For this testing, please provide the gene name and variant information (genomic position, cDNA, and protein information).
GEDi GENE LIST:
METHODOLOGY AND TECHNICAL INFORMATION:
For each panel, the coding regions and splice regions of genes tested are examined. Sanger sequencing is performed to confirm all clinically significant variants and, when necessary, to fill in regions of insufficient coverage.
This testing will not detect large genomic structural rearrangements such as deletions, duplications, inversions, and insertions. Variants in non-coding regions, which are outside of the splicing regions and not specifically targeted, will not be analyzed. Additionally, genetic variants present in genes not known to be associated with retinal degeneration may not be detected by this testing method. Research-based testing is available for detecting these types of genetic alterations.
ORDERING TESTING:
Genetic testing can only be ordered by a medical professional.
Please complete the updated Test Requisition.
This completed form MUST accompany all specimens (hard copy). The Test Requisition includes the following:
- Patient, testing, and sample information (Pages 1-2)
- Consent form (Page 3)
- Billing information (Page 4)
SPECIMEN AND SHIPMENT REQUIREMENTS:
Blood Specimens are the preferred specimen type for testing.
Volume for children: 5-8 mL of whole sterile blood collected in EDTA (lavender top) tube.
Volume for adults: 8-10 mL of whole sterile blood collected in EDTA (lavender top) tube.
Labeling: Tubes should be labeled with the patient’s name, date of birth, and/or ID number.
Shipping: Specimens should be shipped at room temperature and by overnight delivery with an arrival of Monday-Friday. Samples should be received within 48 hours of collection. NO WEEKEND DELIVERY. Samples can be refrigerated before shipping. DO NOT FREEZE BLOOD. In hot weather, a cool pack can be used. Shipping costs are the responsibility of the sender.
SAMPLES SHOULD BE SHIPPED TO THE FOLLOWING ADDRESS:
Ocular Genomics Diagnostic Laboratory
Massachusetts Eye and Ear Infirmary
243 Charles Street, Room 566A
Boston, MA 02114
PRICING:
| Test | Price | CPT Code |
| GEDi-R | $2850.00 | 81434* |
| GEDi-O | $1650.00 | 81479 |
| GEDi-S | $1250.00 | 81479 |
| Validation/Segregation Testing | $500.00 | 81403 |
